Adam Krieger, ITiMS Co-Mentors: Nina Lin, PhD and Bradley Cardinale, PhD
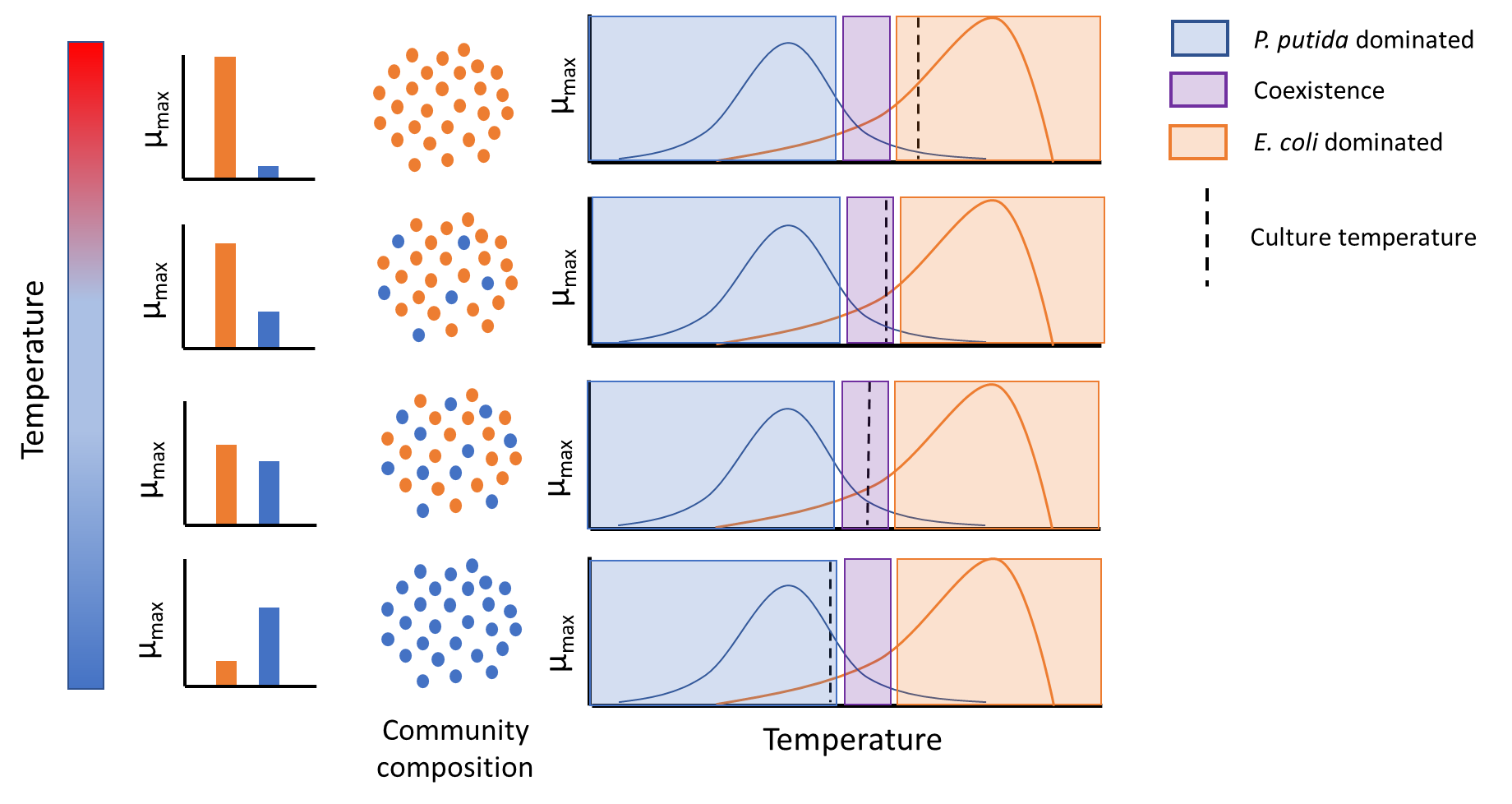
I am interested in the diverse ways that residents of microbial communities communicate and coordinate with each other to produce interesting population level behavior. One way to approach this question is through exploring the mechanisms of maintenance of species diversity from an ecological perspective. The question of exactly what mechanisms enable coexistence of species in a community has been a central theme in ecological research for over a century. Recent efforts to synthesize research in this area have demonstrated that whether or not two or more species can coexist is a function of the magnitude of the difference between the effective growth rates (relative fitness differences or RFDs) of the species and the difference between the niches utilized by each species (niche differences or NDs). A difference between growth rates is a destabilizing factor and will cause populations to diverge into competitive exclusion, whereas niche differences are stabilizing and allow species to coexist. In theory, if the magnitude of the niche differences are greater than the magnitude of the growth rate differences, species will be able to coexist, and vice versa. While this argument makes intuitive sense, ITiMS fellows such as myself are interested in probing similar phenomena quantitively. In order to do so it is necessary to be able to quantify niche differences (a broad concept encompassing a variety of interactions such as nutrient competition, crossfeeding, chemical antagonism, etc.) in a single value that can be meaningfully compared to a growth rate difference so as to be able to predict coexistence or competitive exclusion. I am working with my ITiMS co-mentor Prof. Brad Cardinale to adapt mathematical techniques capable of quantifying relative fitness difference (growth rate differences) and niche differences which have been pioneered by his lab to my experimental system of two bacteria, Psuedomonas putida and Escherichia coli. I am specifically interested in the effect that changing temperature has on modifying relative fitness differences and niche difference in the context of climate change.
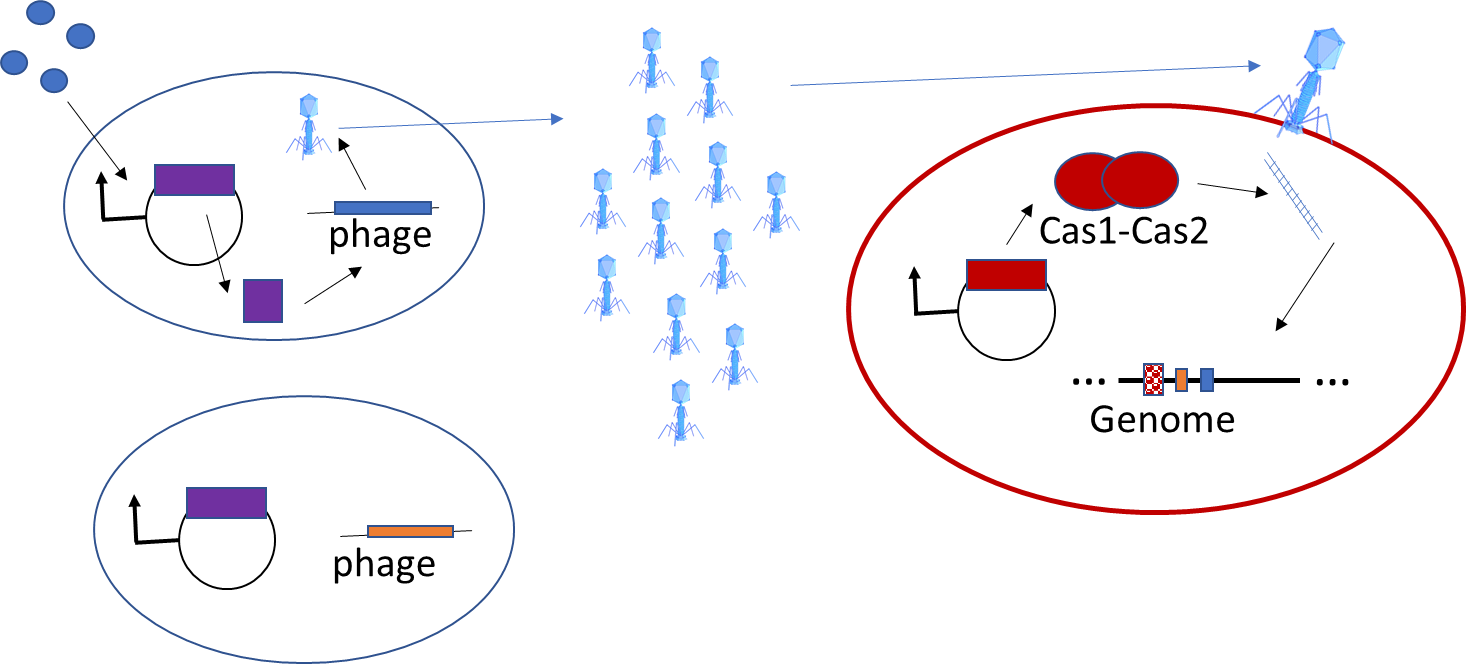
Microbes in communities are also capable of exchanging a variety of different forms of information which can induce a variety of biological behavior. I am working on a second project which will demonstrate biosensing and biomemory in a bacterial community using DNA as a means of encoding information and bacteriophage infection as a means of information transfer between cells. The community contains two specific sub-populations, the biosensing population and the biomemory population. The biosensing population is made up of cells which harbor both a novel genetic circuit under control of a ligand sensitive transcription factor and a prophage. The circuit is designed such that in the presence of the inducing ligand, the gene product of the circuit will activate the prophage to enter its lytic cycle. Each prophage encodes a unique DNA barcode that is specific to the particular ligand to which its particular transcription factor is sensitive, thereby encoding information about which chemical to which the community was exposed. Barcoded phage are then released into the culture media in response to addition of their corresponding ligand and subsequently infect the biomemory population of cells. These cells are engineered to express the CRISPR Cas adaptation machinery complex Cas1-Cas2. Upon injection of phage DNA (containing the barcode) by the virion, the Cas1-Cas2 complex targets the phage DNA, excises the barcode, and inserts it into the genomic CRISPR locus as a new spacer. This acts as a genomic memory of the sensing event (that the community was exposed to the chemical ligand of the transcription factor) and can be faithfully passed from generation to generation. Each biosensing cell can sense a difference ligand, but each sensing event is remembered in a central memory system. Because new spacers are always added to the same end of the CRISPR locus, the sequence of spacers in the locus encodes temporal information about the order of addition of the chemical signals. To read out which chemicals the community has been exposed to, it is possible to simply sequence the CRISPR locus and read out the different barcodes present as spacers. This type of synthetic biosensing and biomemory system is desirable for biosensing platforms solicited by DARPA.