ITiMS provided funds and support to achieve this successful manuscript! Pellitier, P.T., Zak, D.R. and Salley, S.O., 2019. Environmental filtering structures fungal endophyte communities in tree bark. Molecular ecology.
Environmental filtering structures fungal endophyte communities in tree bark
by Peter Pllitier
Introduction
Fungal endophytes comprise a critical and ubiquitous component of the plant microbiome, forming cryptic asymptomatic infections in virtually all above- and below-ground plant tissues. Paradoxically, other fungi frequently isolated from asymptomatic plant tissue are placed in taxonomic groups typically associated with saprotrophs or plant pathogens, leading to the suggestion that some fungi recovered living as endophytes also occur as a range of other lifestyles. Fungal communities in tree bark are particularly understudied, and I present the first molecular sequencing enabled characterization of these communities. Bark fungi may hold underappreciated ecological significance, as they inhabit one of the largest plant surfaces by area and reside in plant tissue that is frequently the site of devastating insect and associated pathogen attack (i.e., western bark beetle, chestnut blight and Dutch elm disease)
This work explored several fundamental questions concerning the community composition and diversity of inner bark fungi spanning a range of common temperate tree species. Foremost, I sought to determine the identity and diversity of fungi inhabiting these tissues. Additionally, I tested the hypothesis that tree bark acts as an environmental filter structuring the community membership of inner bark fungi. Finally, I explored whether chemical variation in tree bark can account for differences in fungal community composition.
Research Methods and Results
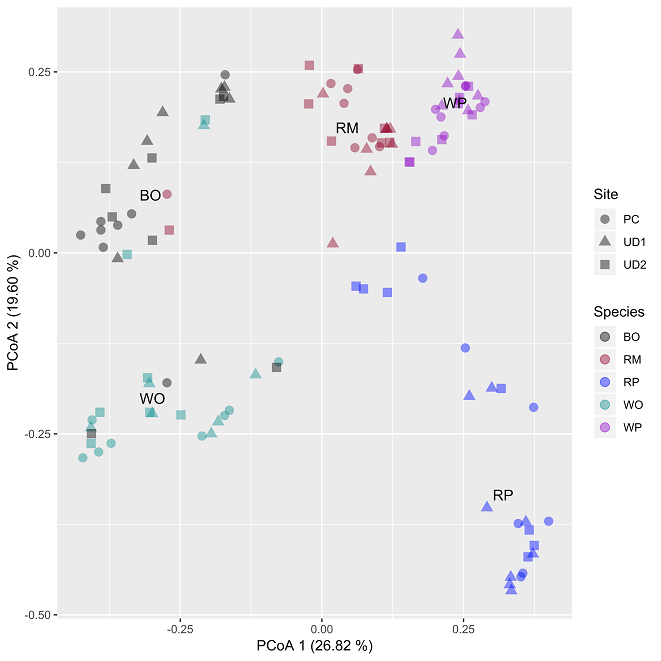
Three sites were identified in Manistee National Forest, Michigan, USA; five dominant tree species were sampled in replicate across all sites (Figure 1). A novel DNA extraction procedure was developed to isolate high quality double-stranded DNA suitable for Polymerase Chain Reaction. Sequences were processed using QIIME and chemical components of bark were assessed using a range of laboratory methods. I found that inner bark fungal communities were highly diverse (mean of 117–171 OTU per tree) and dominated by Ascomycete fungi living asymptomatically as putative endophytes. I also found that each of the five-tree species host unique assemblages of inner bark fungi and that angiosperm and gymnosperm host significantly different fungal communities (Figure 2). This work highlights that inner tree bark harbor nonrandom fun gal assemblages and that these communities are an underappreciated and potentially ubiquitous component of the plant microbiome.
Future Steps
The role of these inner bark communities in plant defense endophytes remains an intriguing area of research. Studies exploring the role of inner bark fungi in deterring chestnut blight, or Dutch Elm disease is an urgent area of research that I plan on pursuing using my newly developed DNA extraction procedures. Additionally, the unique inner bark fungal assemblages found in each tree species may impact subsequent decay dynamics. The role of inner bark fungi as immediate colonizers of dead plant tissue is plausible, especially given the potentially saprotrophic capacity of some of the dominant fungi found here.
Figure 1. Bark sampled to the depth of the vascular cambium. Reading left to right: a. Red pine, b. White pine, c. White pine and Red Pine, d. Red Maple, e. White Oak
Figure 2: Principle coordinates analysis (PCoA) based on Bray–Curtis distance matrices. Fungal OTUs exclude lichenized fungi. Individual points represent inner bark fungal communities from individual trees (colours) and sites (shapes). Plotted tree species labels represent centroid locations. Differences among tree species explain ~20 times more variation in fungal community composition than does site. BO, black oak; RM, red maple; RP, red pine; WO, white oak; WP, white pine