Paper resulting from this project: https://doi.org/10.1111/1462-2920.15352
"Sulfide alters microbial functional potential in a methane and nitrogen cycling biofilm reactor"
Jeseth Delgado Vela, Laura A. Bristow, Hannah K. Marchant, Nancy G. Love, Gregory J. Dick
First published: 09 December 2020, Environmental Microbiology
***
Jeseth Delgado Vela received a $10,000 ITiMS mini-grant for her project:
Linking Sulfur and Nitrogen Cycles in Wastewater Treatment Processes using Gene-Based Modeling of a Membrane Aerated Biofilm Reactor
Co-advisors: Gregory J. Dick and Nancy G. Love
INTRODUCTION
Excessive nutrients in U.S. freshwaters lead to economic losses of 2.2 billion dollars annually due to losses in biodiversity, impaired water bodies used for drinking and recreational purposes, and reduced waterfront property values(1). Alongside increasingly stringent nutrient regulations, utilities and cities are facing uncertain energy costs and impending regulations on greenhouse gas emissions. One potential alternative to conventional wastewater treatment is anaerobic treatment processes that enhance energy recovery from wastewater by eliminating aeration, the most energy intensive aspect of wastewater treatment, and converting organic matter into biogas, an energy source(2). However, anaerobic processes do not remove nitrogen or phosphorus, thus nutrient removal following anaerobic treatment is necessary. Although anaerobic treatment presents some opportunities for wastewater treatment, it needs to be implemented with technologies that treat the nitrogen in order to support widespread adoption of this technology.
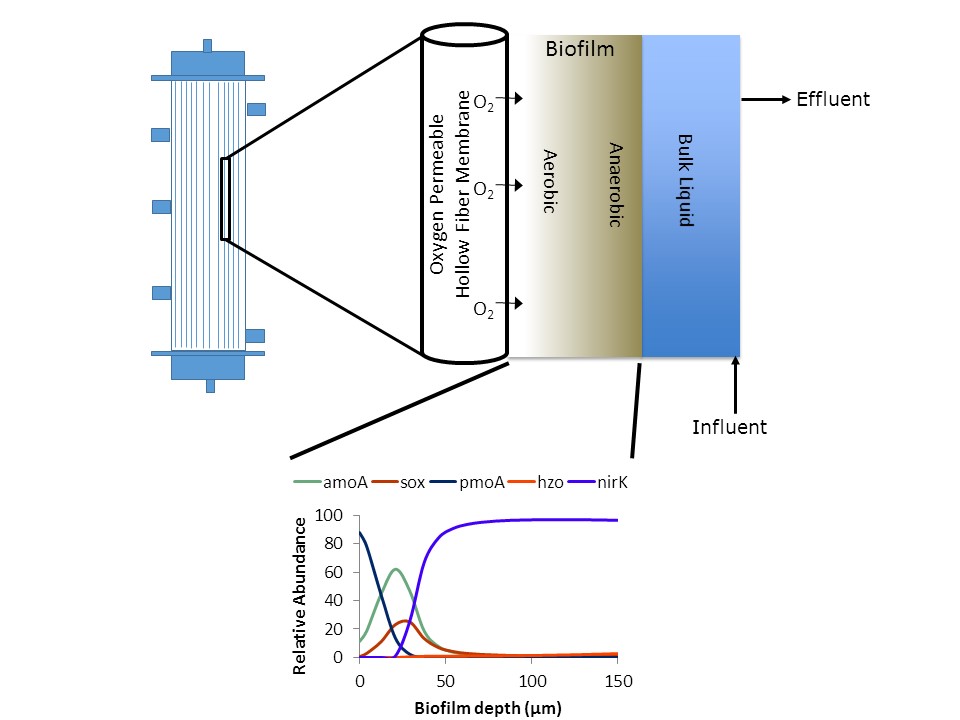
The membrane aerated biofilm reactor (MABR) is suitable for biological nitrogen removal (the conversion of ammonium to nitrogen gas) from anaerobically treated domestic wastewater. MABRs are an emerging wastewater treatment technology and use membranes to supply diffused oxygen to a bioreactor and to support a biofilm that removes nitrogen. An ideal anaerobic effluent contains low amounts of residual organics, because more of the carbon in the wastewater was converted to biogas during the anaerobic process. Consequentially, conventional heterotrophic denitrification does not remove sufficient nitrogen and autotrophic nitrogen removal from anaerobic effluents is needed. One such pathway is nitrogen removal via sulfide oxidation. Given the multi-redox biofilm environment in an MABR biofilm, sulfide would not necessarily be aerobically oxidized in the reactor. As the wastewater treatment industry moves towards reducing aeration energy demands and creating treatment environments with low dissolved oxygen, simultaneous sulfur and nitrogen cycling may play an important role in treatment plant performance. This research is focused on studying the impact of sulfide on nitrogen removal in a multi-redox MABR system.
The link between the sulfur and nitrogen cycles in wastewater treatment plants is not well-understood. Some studies have evaluated sulfide inhibition of complete nitrification (ammonium oxidation to nitrate)(3–5) or using enrichment cultures(6). We have evaluated sulfide-inhibition of ammonium oxidizing microorganisms (AOM) and nitrite oxidizing bacteria (NOB) separately using full-scale wastewater microbial communities. Beyond evaluating sulfide inhibition of nitrification, a better understanding of how sulfur impacts simultaneous nitrification and denitrification is needed, especially in multi-redox biofilm systems such as the MABR. To do so we can adopt techniques used by researchers studying environmental redox gradients such as marine oxygen minimum zones where we are beginning to understand how microbial cycling of sulfur and nitrogen are linked. High throughput whole genome sequencing (metagenomic) studies of oxygen minimum zones in the deep ocean have shown that cycling of sulfide can help drive nitrogen removal(7). We hypothesize that sulfur cycling could also drive nitrogen removal in the MABR system. This research will build upon an existing one-dimensional biofilm model to
include sulfur based biogeochemical interactions relevant to the MABR system to evaluate whether cryptic cycling of nitrite and sulfide occur in MABR biofilms.
RESEARCH OBJECTIVES
We are currently operating a lab-scale MABR that treats synthetic anaerobic effluent in the environmental biotechnology laboratory at the University of Michigan. In addition, we have developed a one-dimensional biofilm model of the MABR reactor. We have also evaluated the extent to which sulfide inhibits nitrifying cultures, and will be using the experimentally derived sulfide inhibition parameters in the existing biofilm model. By coupling experimentation with modeling, we have the ability to generate new hypotheses using modeling and test the hypotheses in the laboratory. The specific aims of this research are:
Aim 1: Incorporate sulfur cycling into current biofilm model, and convert population-based biofilm model to a gene-based biofilm model.
Aim 2: Calibrate and validate the model using both molecular methods and microsensor studies in the biofilm.
Aim 3: Conduct a model-based investigation of the membrane aerated biofilm to understand the extent to which sulfur impacts nitrogen removal in the biofilm.
APPROACH
Incorporate sulfur cycling into current biofilm model, and convert population-based biofilm model to a gene-based biofilm model.
We will build off of ongoing work where we evaluated sulfide inhibition of nitrifiers using microbial communities from two different full-scale wastewater treatment processes. We determined the inhibition constants for NOB and AOM associated with sulfide. Our results show that NOB are more sensitive to sulfide than AOM (Figure 1). From these experiments, we have a better understanding of the sulfide inhibition constants and the uncertainty range for sulfide inhibition of nitrifiers. These inhibition constants will now be applied to an existing one-dimensional biofilm model developed using the popular biofilm modeling software AQUASIM(8). This existing biofilm model incorporates many species relevant to nitrogen removal from anaerobic effluents: methane oxidizing, nitrifying, and denitrifying bacteria. However, sulfur inhibition of nitrification, aerobic sulfur oxidation, and sulfide-based denitrification are not currently evaluated in the existing biofilm model and the equations governing these processes will be incorporated into the biofilm model.
The existing model is a population-based model where different microbial populations are being modeled using Monod dual-substrate kinetics. Existing wastewater treatment process models such as our population-based biofilm model could be strengthened with the addition of genetic information. For example, sulfide based denitrification is often incomplete(9–11), releasing nitrous oxide or nitric oxide. Therefore, we cannot assume populations have a complete pathway. By modeling the enzymatic processes occurring within the cells, instead of the full population, we may improve the predictive capacity of our existing process models. Given the rapidly expanding sequencing technologies, gene-based models could be calibrated and validated using high throughput, whole community sequencing data (metagenomics). This task will adapt methods from a previously developed gene-based model(12) to incorporate genetic data into our existing model.
Calibrate and validate the model using both molecular methods and microsensor studies in the biofilm.
Integrating molecular data and modeling results is novel in the wastewater treatment field and doing so can help elucidate the cycling of chemicals mediated by microbes, which cannot be measured in the bulk liquid of the reactor. Model sensitivity analysis will be conducted using a normalized absolute-relative sensitivity function(8). This function creates a linear approximation of a function describing the relationship between the state variable (model output) and the kinetic and stoichiometric parameters (constant variables) for each simulation time point (day). Using the normalized absolute-relative sensitivity function we will compare the model sensitivity across many variables because the sensitivity is defined as the relative change in concentration resulting from a doubling in the parameter value. AQUASIM then averages the sensitivity for each day to rank the sensitivity of constant variables. Parameters that rank high on the sensitivity analysis have a high degree of impact on the simulated effluent total nitrogen concentrations and are thus more important to identify using experimental data. Critical parameters will be calibrated using bulk liquid reactor chemical data, metagenomics sequencing data from the biofilm, and, drawing on existing expertise in the geomicrobiology laboratory(13), microsensor profiles of sulfide, pH, dissolved oxygen and nitrous oxide through the biofilm. The metagenomic sequencing analysis will use sequence assembly, read mapping, and annotation of key functional genes as described previously(14). The parameter estimation will be conducted on the parameters that have the greatest impact on effluent total nitrogen by minimizing the weighted sum of the difference between the steady-state experimental reactor data and the simulated effluent data using the simplex technique, a numerical minimization algorithm suitable even when initial parameter values are poorly defined.
Conduct a model-based investigation of the membrane aerated biofilm to understand the extent to which sulfur impacts nitrogen removal in the biofilm.
Having a calibrated and validated model, we can test the extent to which sulfur is impacting nitrogen removal from the membrane aerated biofilm reactor. This investigation will help elucidate if sulfide can drive nitrogen removal by serving as an electron donor for denitrification, if sulfide is increasing nitrous oxide emissions due to incomplete denitrification, or if sulfide is inhibiting nitrification. These model outputs will help generate hypotheses on the extent to which sulfur cycling is driving nitrogen removal in the membrane aerated biofilm. We will simulate a range of different types of wastewater, taken the variability we have observed of sulfide concentrations in effluents from mainstream anaerobic technologies(15).
BROADER IMPACT
Integrating biomolecular data into wastewater treatment process models has not been done to the extent to which we are proposing. This type of model based investigation will not only elucidate the impact of sulfur on nitrogen removal processes, but can also impact the extent to which these types of gene-based models can be widely applied to understand biogeochemical cycling in other environments. Given our increasing understanding of the extreme microbial diversity on earth, and the extent to which very closely related populations can be functioning differently in the environment, a gene-based modeling investigation could give us a more complete picture of how wastewater treatment processes are functioning. We will have a greater appreciation for how the nitrogen and sulfur cycles are linked in wastewater treatment processes, and how chemical constituents are cycled within a biofilm. In addition, this research is a true integration of modeling and laboratory-based investigations, as our laboratory studies are informing our model both by providing us with kinetic constants and by serving as an important tool for calibrating and validating our model. Our model will also inform future studies on sulfide-based denitrification in wastewater treatment and within nitrifying and denitrifying biofilms.
REFERENCES
(1) Dodds, W. K.; Bouska, W. W.; Eitzmann, J. L.; Pilger, T. J.; Pitts, K. L.; Riley, A. J.; Schloesser, J. T.; Thornbrugh, D. J. Eutrophication of US freshwaters: analysis of potential economic damages. Environ. Sci. Technol. 2008, 43 (1), 12–19.
(2) McCarty, P. L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer--can this be achieved? Environ. Sci. Technol. 2011, 45 (17), 7100–7106.
(3) Bejarano Ortiz, D. I.; Thalasso, F.; Cuervo López, F. D. M.; Texier, A. C. Inhibitory effect of sulfide on the nitrifying respiratory process. J. Chem. Technol. Biotechnol. 2013, 88 (7), 1344–1349.
(4) Joye, S. B.; Hollibaugh, J. T. Influence of Sulfide Inhibition of Nitrification on Nitrogen Regeneration in Sediments. Science (80-. ). 1995, 270 (5236), 623–625.
(5) Zhou, Z.; Xing, C.; An, Y.; Hu, D.; Qiao, W.; Wang, L. Inhibitory effects of sulfide on nitrifying biomass in the anaerobic-anoxic-aerobic wastewater treatment process. J. Chem. Technol. Biotechnol. 2014, 89 (2), 214–219.
(6) Bejarano-Ortiz, D. I.; Huerta-Ochoa, S.; Thalasso, F.; Cuervo-López, F. de M.; Texier, A. C. Kinetic Constants for Biological Ammonium and Nitrite Oxidation Processes Under Sulfide Inhibition. Appl. Biochem. Biotechnol. 2015, 177 (8), 1665–1675.
(7) Canfield, D. E.; Stewart, F. J.; Thamdrup, B.; Brabandere, L. De; Delong, E. F.; Revsbech, N. P.; Ulloa, O.; De Brabandere, L.; Dalsgaard, T.; Delong, E. F.; et al. A cryptic sulfur cycle in oxygen-minimum-zone waters off the Chilean coast. Science 2010, 330 (6009), 1375–1378.
(8) Reichert, P. AQUASIM - A Tool for Simulation and Data Analysis of Aquatic Systems. Water Sci. Technol. 1994, 30 (2), 21–30.
(9) Sorensen, J.; Tiedje, J. M.; Firestone, R. B. Inhibition by Sulfide of Nitric and Nitrous-Oxide Reduction by Denitrifying Pseudomonas-Fluorescens. Appl. Environ. Microbiol. 1980, 39 (1), 105–108.
(10) Dalsgaard, T.; De Brabandere, L.; Hall, P. O. J. Denitrification in the water column of the central Baltic Sea. Geochim. Cosmochim. Acta 2013, 106 (January 2003), 247–260.
(11) Schonharting, B.; Rehner, R.; Metzger, J. W.; Krauth, K.; Rizzi, M. Release of Nitrous Oxide (N2O) from denitrifying activated sludge caused by H2S-Containing Wastewater: Quantification and Application of a new mathematical model. Water Sci. Technol. 1998, 38 (1), 237–246.
(12) Reed, D. C.; Algar, C. K.; Huber, J. a.; Dick, G. J. Gene-centric approach to integrating environmental genomics and biogeochemical models. Proc. Natl. Acad. Sci. 2014, 1–6.
(13) Klatt, J. M.; Meyer, S.; Häusler, S.; Macalady, J. L.; de Beer, D.; Polerecky, L. Structure and function of natural sulphide-oxidizing microbial mats under dynamic input of light and chemical energy. ISME J. 2015, 1–13.
(14) Anantharaman, K.; Breier, J. a; Dick, G. J. Metagenomic resolution of microbial functions in deep-sea hydrothermal plumes across the Eastern Lau Spreading Center. ISME J. 2015, 10 (1), 225–239.
(15) Delgado Vela, J.; Stadler, L. B.; Martin, K. J.; Raskin, L.; Bott, C.; Love, N. G. Prospects for Biological Nitrogen Removal from Anaerobic Effluents during Mainstream Wastewater Treatment. Environ. Sci. Technol. Lett. 2015, 2 (9), 233–244.