James Tan received a $5,000 mini-grant from ITiMS for this project.
Elucidation of metabolic interactions in a naturally-derived Microcystis consortium
Co-researchers: Dr. Derek Smith, Dr. Sara Rivera
Advisors: Xiaoxia “Nina” Lin, Evan Snitkin, Gregory Dick
Generating toxic blooms, Microcystis aeruginosa, an aquatic cyanobacterium, affects freshwater systems globally (1), including Lake Erie. In aquatic systems, the metabolic interactions between photosynthetic cyanobacteria and their associated bacterial partners are critical for their proliferation, but have remained understudied and elusive. Microcystis exists as aggregates, which are referred to as colonies. The physically-associated microbial consortia on these Microcystis colonies – referred to as the phycosphere (2) – are believed to have interactions with their host cells, facilitated by their close proximal distance (3). Hypothesized interactions include the exchange of fixed carbon substrate from Microcystis to the phycosphere (4), the synthesis and exchange of vitamins (5), or the detoxification of certain reactive oxygen species (6) by the phycosphere for Microcystis. The elucidation of these specific interactions will provide fundamental insights into Microcystis’ ability to proliferate and its resilience during bloom seasons.
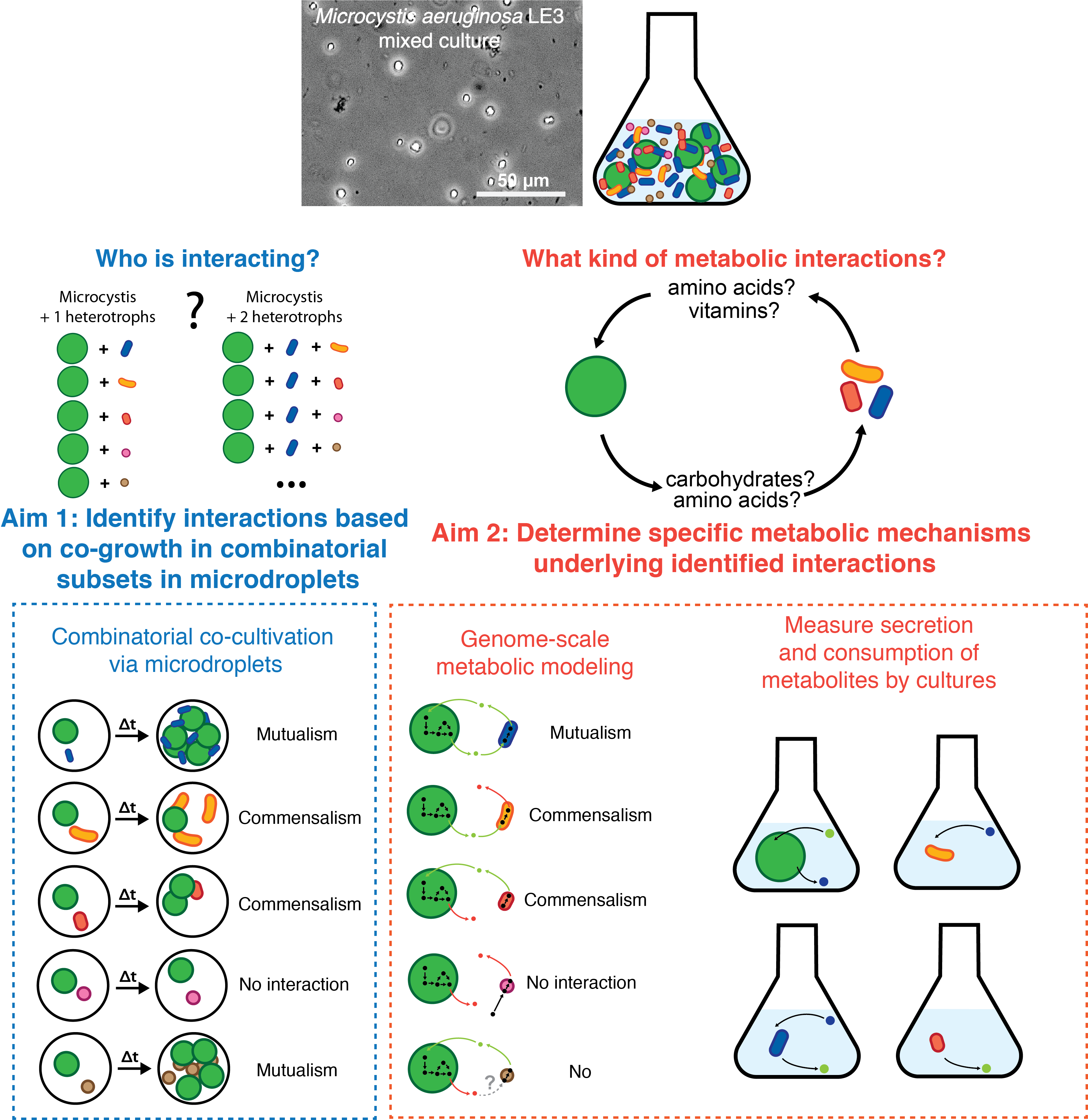
We are studying these interactions by the use of a mixed culture of M. aeruginosa LE3. This mixed culture of M. aeruginosa was initially cultivated in 1996 (7), harboring the Microcystis strain LE3 and around 10 other heterotrophic bacteria in high abundance, suggesting intimate mutualistic relationships. While LE3 provides a system of study to interrogate critical interactions between Microcystis and its heterotrophic partners, it is unknown if M. aeruginosa LE3 has metabolic dependencies on its heterotrophic partners and what metabolites may be exchanged. We are applying microfluidic droplets, which are sub-nanoliter to microliter scale water-in-oil emulsions, as miniaturized bioreactors for microbial cultivation, rendering ultra-high throughput investigation (8) trivial. The small size of microdroplets allows us to encapsulate small subsets of the LE3 consortium at massively high-throughput rates, decomposing it into random combinatorial subsets of species combinations. Using high-throughput droplet barcoding to perform quantitative 16S taxonomic surveys on thousands of droplets, we can determine which combinations of bacteria contribute to M. aeruginosa LE3 growth and which do not.
Aim 1: Identify interactions in the LE3 mixed culture based on co-growth in randomized, combinatorial subsets in microdroplets
To match the high-throughput generation of microdroplet co-cultivation (8,9), we will adapt a droplet platform to incorporate unique sequencing barcodes and an internal standard for quantitative 16S profiling of individual droplets. The system will be tested on cell mixtures of known compositions to benchmark the quantitative capability. After validating the platform, we will apply the method to the LE3 mixed culture to identify which specific random subsets of partners result in Microcystis-heterotroph co-growth.
Aim 2: Determine specific metabolic mechanisms underlying identified interactions
Using constraint-based genome-scale metabolic models (GSMMs) generated from genomes from metagenomically-assembled genomes (MAGs) of the LE3 mixed culture, co-growth or lack of growth can be independently investigated. Metabolic exchanges observed in GSMM that maximize the consistency between co-growth observed with GSMM and the experimental results from the microdroplets will be further investigated. Metabolic exchanges between Microcystis and heterotrophs hypothesized to be of significance can be verified using strains isolated from LE3. Secretion and consumption of these predicted metabolites by isolates with culturing and analytical chemistry can confirm these hypotheses.
References
1. Harke MJ, Steffen MM, Gobler CJ, Otten TG, Wilhelm SW, Wood SA, et al. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae. 2016;54:4–20.
2. Seymour JR, Amin SA, Raina JB, Stocker R. Zooming in on the phycosphere: The ecological interface for phytoplankton-bacteria relationships. Nat Microbiol. 2017;2.
3. Smith D, Tan J, Powers M, Lin XN, Davis T, Dick G. Individual Microcystis colonies harbor distinct bacterial communities that differ by Microcystis oligotype and with time. Environ Microbiol (accepted, 2021).
4. Kehr J-C, Dittmann E. Biosynthesis and function of extracellular glycans in cyanobacteria. Life. 2015;5(1):164–80.
5. Shen H, Niu Y, Xie P, Tao M, Yang X. Morphological and physiological changes in Microcystis aeruginosa as a result of interactions with heterotrophic bacteria. Freshw Biol. 2011;56(6):1065–80.
6. Cory RM, Davis TW, Dick GJ, Johengen T, Denef VJ, Berry MA, et al. Seasonal Dynamics in Dissolved Organic Matter, Hydrogen Peroxide, and Cyanobacterial Blooms in Lake Erie. Front Mar Sci. 2016;3:1–17.
7. Meyer KA, Davis TW, Watson SB, Denef VJ, Berry MA, Dick GJ. Genome sequences of lower Great Lakes Microcystis sp. reveal strain-specific genes that are present and expressed in western Lake Erie blooms. PLoS One. 2017;12(10).
8. Park J, Kerner A, Burns MA, Lin XN. Microdroplet-enabled highly parallel co-cultivation of microbial communities. PLoS One. 2011;6(2).
9. Tan JY, Wang S, Dick GJ, Young VB, Sherman DH, Burns MA, et al. Co-cultivation of microbial sub-communities in microfluidic droplets facilitates high-resolution genomic dissection of microbial ‘dark matter.’ Integr Biol. 2020;12(11):263–74.